|
| Positions for undergraduate and graduate assistants available. |
|
|
| |
Members
Log In |
|
|
|
| Our
Mission |
|
|
NMR is a non-invasive technique to probe the structure and dynamics of molecules and atoms. The acquired NMR spectrum exhibits features such as shift of resonance frequency,
linewidth, and symmetry of lineshape, which are manifestations of perturbations of local magnetic field on the site of the nucleus under surveillance. Combined with theoretical and empirical interpretations,
They inform us the electronic and magnetic structure of materials, or geometric constraints between atoms. Information from NMR can be complementary to those from other spectroscopy techniques, such as X-ray diffraction,
Electron Microscopy (EM). In some situations, NMR can be the only effective tool applicable. In this lab, we utilize NMR as the major force to characterize the properties of materials, in combination with other spectroscopy methods and theorectical calculations and simulations.
|
| |
| Electronic and Magnetic Properties of Catalytic Nano Particles |
|
|
Nano particles of many transition metals are widely used to catalyze chemical reactions. As chemical
properties are dominated by the valence electrons, it's conceivable that the enhancement in catalytic performance is
associated with the altered band structures due to the unique physical properties of nano particles that approach microscopic
scales. However, significant challenges pose against the extraction and interpretation of the relations between the two. We take
advantage of the non-invasive nature of NMR to probe the electronic and magnetic properties associated with band structures and
identify the effects of the physical properties of such nano particles on enhancements of catalytic behaviors. |
| |
| Structure and Dynamics of self-Assembled biomolecules |
|
|
Solution NMR has been highly successful in characterization of the structure and dynamics of molecules tumbling in solutions,
such as polymers or proteins. However, there are a large group of biomolecules that aggregates into large complexes and precipitate out of the solution, therefore
they become "invisible" to solution NMR techniques. Many of these entities are associated with pathogens or diseases that impact human society dearly, examples are
capsid shells in viruses, amyloid plaques observed in many neurodegenative disease such Alzheimer disease, or the famous mad-cow disease. It is of great value to
resolve the structures and dynamics of such aggregates in order to decipher the assembly mechanism and provide insight to conquer these terrible diseases. Solid state NMR (ssNMR)
is one of the few techniques applicable to these subjects. We are actively developing and applying ssNMR methods to study the capsid protein assembly of HIV-1 virus and the prions
present in yeast Saccharomyces Cerevisiae. We are also working out innovative models for simulations of self-assembly.
Self-assembly is an intriguing phenomenon exhibited by many chemical and biological systems, for example, prions and virus capsid proteins. Usually, a large number of molecules are involved, and it is impossible to simulate the process with full-atom representations with current computation
resources. Coarse-grain modelling are often adopted where simple geometric objects such as spheres or polygons are used. In this case, a boost of simulation speed is achieve at the expense of molecular structures. Insights into basic principles can be obtained with such simplified models. However,
it is in general difficult to relate the simulation to experimental observations. Here I designed a novel coarse grain model to capture the overall geometry of the protein molecules, as shown in Figure 1.
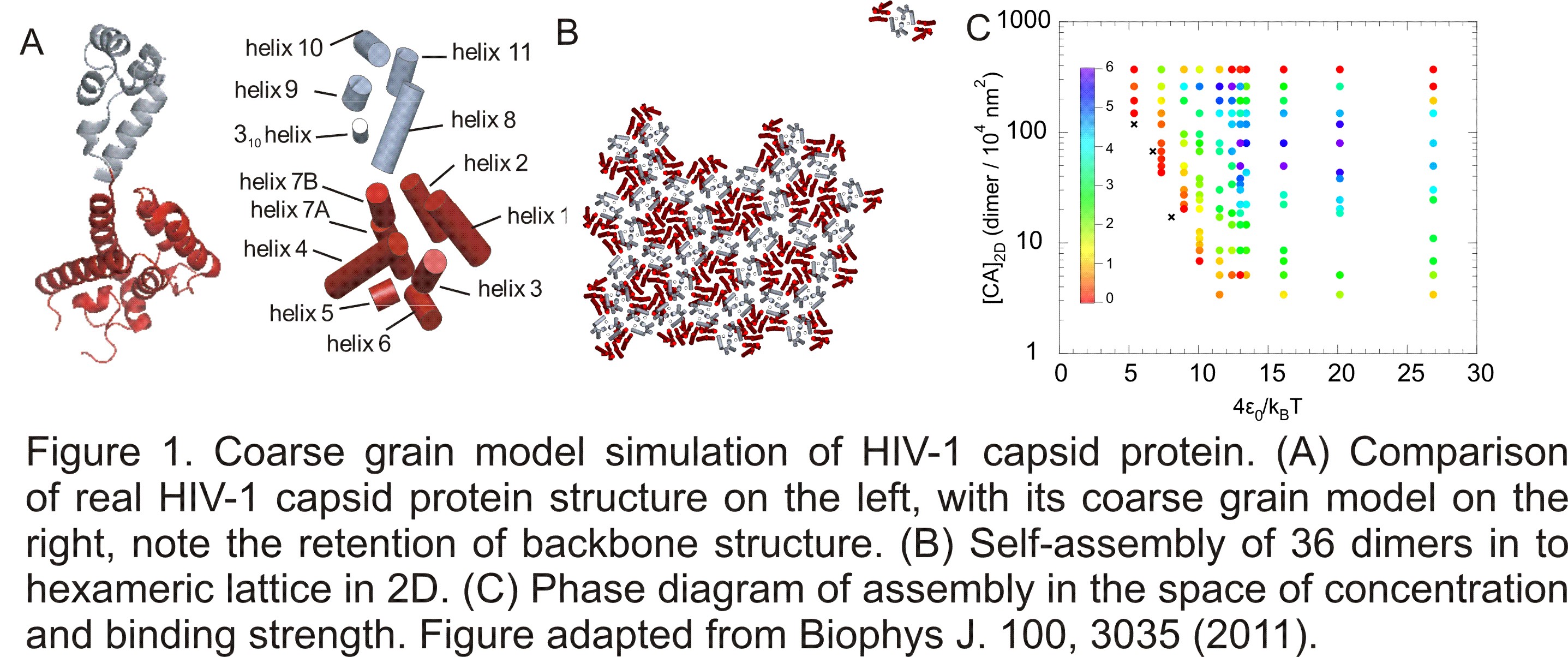
Monte Carlo simulation with this model applied successfully to simulate the self-assembly
of HIV-1 capsid protein in 2D (Biophys. J. 100, 3035 (2011)). The movie compiled from this work is shown below.
|
| Structure and Dynamics of Antimicrobial peptides (AMPs) |
|
|
AMPs are short polypeptides capable of disruption of lipid membrane. They are so called “amphipathic molecules”,
include both hydrophobic and hydrophilic amino acids, which intend to interact with the charged head groups and long hydrophobic chains
of molecules in lipids. Generally speaking, AMPs interact with the lipid bilayers to increase the ion permeability and changes the electrochemical
potential across the membrane, thus induce the killing of the cells. In addition, many AMPs also exhibit selectivity of toxicity against bacteria than
eukaryotic cells. Therefore, they are promising candidates for antibiotics to open new frontlines in the battle against the bacterial resistance in
medicine. More than 800 AMPs have been identified and documented. However, the mechanism of their function and selectivity towards bacteria is still debated.
We will use ssNMR to study the structure and dynamics associate with variants of AMPs, and look for clues or answers to this million dollar question. |
| |
|
| |
|
|
| |
|
©2011 Bo's NMR lab. All Rights Reserved.
|
|