There are many fundamental microscopic issues that are important in metal-matrix composites, that typically deal with the chemistry of the interface between the alloy matrix and the ceramic, which may be in the form of a particle, fiber, or whisker. Typical concentrations of ceramic range from 10-20%, with particle sizes ranging from a few microns to more than 100 microns in diameter.
We have begun some spectromicroscopy studies of SiC fiber reinforced Ti alloys, which illustrate the kind of information one can get, by studying the changes in the chemistry of the matrix-ceramic interface. Certain failure modes of the composite will be determined by the adhesion of the metal matrix to the fiber surface. Also, the interaction of the metal with the fiber as a function of temperature and time is a major factor in determining the life cycle of a composite, and its suitability for processing.

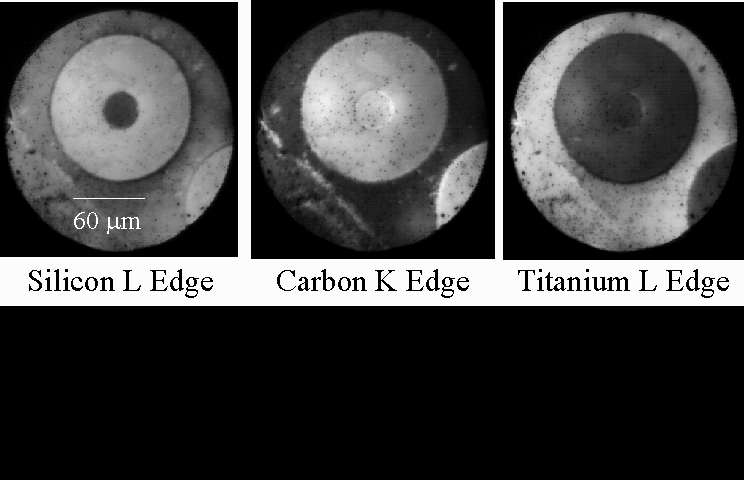
A sequence of XPEEM images of SiC reinforced Ti alloy is shown in the figure above, from recent work using the PRISM x-ray emission microscope at the SpectroMicroscopy Facility. These images are taken at certain photon energies, which create good chemical contrast between different regions of the composite. The fiber itself is rather complex, consisting of a central core region of pyrolitic carbon, a region of SiC, and an outer layer consisting mostly of carbon, with some small SiC inclusions. All of these regions are easily distinguished in the x-ray micrographs, and the elemental composition of the various regions can be used to preferentially enhance the emission from one area or the other. This is done by selecting a photon energy near the absorption edge of Si, C, or Ti, respectively.
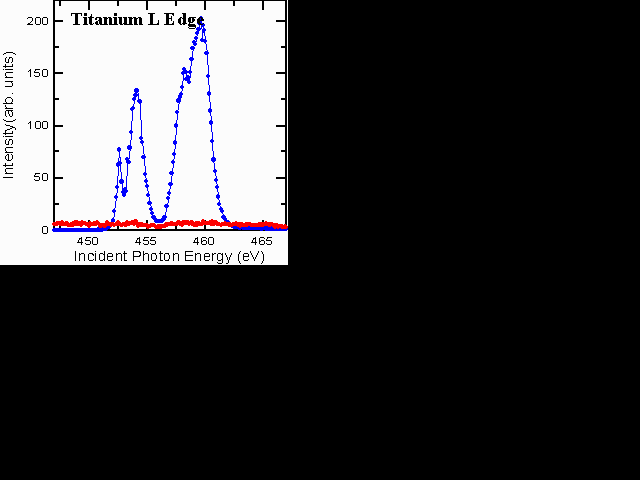
We get chemical information from small areas of the sample using "microXANES." XANES stands for x-ray absorption near-edge structures, and is an x-ray absorption fine-structure spectroscopy (XAFS). It tells us about the chemical state of the material. The microXANES spectra from each region of the metal-matric composite contain sharp structures that aid in determining the chemical composition. For example, the C K-edge spectra are very different for C in the central core of the fiber, in the SiC region, and in Ti, reflecting the bond changes from graphitic, to carbidic species. The Ti L-edge spectra from the fiber (red) and the matrix (blue) are shown below. No Ti is found in the fiber region for this sample.
Interesting changes occur in these composites when they are heat treated. The heat treatment causes reactions to take place between the fiber and the Ti alloy matrix. Concentration profiles develop indicating substantial migration of Si, C, and Ti, from one region of the sample to another. The diffusion is very nonuniform, with different behavior for all three elements.
Metal-matrix composites like these have been studied by other techniques, including transmission electron microscopy with energy-loss spectroscopy (TEM-EELS), electron probe x-ray spectroscopy and scanning Auger microscopy. The unique advantage we can offer is the enhanced ability to detect the chemical state of reaction products at interfaces, directly, by XANES and XPS spectroscopy. For example, one study of SiC-Al MMC's found an increase of oxygen signals in various parts of the sample, but could not directly say what was happening: aluminum oxide formation, hydroxide, silicon oxide, or organic oxygen? We can answer such questions easily with the chemical-shift and fingerprinting of XANES and XPS.
(last updated 27 December 1995 by B. Tonner)